Abstract
Iron is required for hemoglobin synthesis and for the maturation of erythrocytes in the marrow. Disorders of iron delivery to erythropoietic tissues cause anemia. Erythroferrone (ERFE) is a marrow-derived hormone that coordinates the availability of iron for developing red cells. ERFE acts by suppressing hepatic production of the iron regulator hepcidin, thereby making iron from body stores and from the diet accessible for use by erythroblasts. The ERFE mechanism of action is poorly understood. Here, we present a structure-function study of ERFE, elucidating how its structural domains enable its important biological function.
Suppression of hepcidin by ERFE is thought to be mediated by the sequestration of bone morphogenetic proteins (BMPs) that act through the SMAD pathway to activate hepcidin transcription. The structural elements of the ERFE protein include a signal sequence to direct it for extracellular trafficking, an N-terminal extended section with very few structured segments, a C-terminal TNFα-like globular head, and multiple glycosylated residues. We evaluated the contributions of these features to the functional activity of the hormone as well as to its ability to bind BMPs. In our cell-based functional assay, the Hep3B human liver cell line is treated with human ERFE or its mutated variants and assayed by qPCR for hepcidin mRNA abundance. We measure the direct binding interactions between BMPs and ERFE variants using surface plasmon resonance. We found that the N-terminal region of the molecule-just over 100 residues of the 354 amino acid protein-was sufficient to bind BMPs and suppress hepcidin; the larger C-terminal region does neither. Moreover, we found that when this "active domain" of ERFE is produced in bacterial cells it is as active as from mammalian cell lines, even without refolding. The amino acid residues modified by glycosylation are located in the C-terminus of the protein and are thus likewise unnecessary for biological function or BMP binding, although they may modify the stability and oligomeric state of the protein.
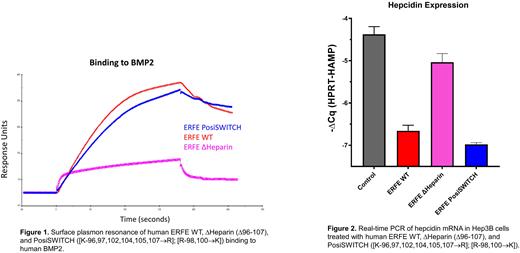
Next, we investigated the contributions of regions and individual residues in the putative active domain of human ERFE. Within this domain, we identified several evolutionarily conserved features including a hydrophobic region from residues 81 to 86 and a nearby highly positively charged tract spanning residues 96 to 107. We performed targeted mutagenesis of these regions and found both features to contribute to ERFE biological activity. In both areas, the targeted substitution of a single amino acid is sufficient to ablate hepcidin suppression, suggesting the involvement of multiple ERFE domains in BMP binding. We determined that in addition to its importance for functional activity, the positively charged region also mediates the binding of ERFE to heparin. Disruption of the heparin-binding domain also interfered with BMP binding (Figure 1) and hepcidin suppression (Figure 2). Restoration of heparin binding via non-native cationic amino acids reestablished both BMP binding and functional activity, indicating for the first time the importance of salt bridges in ERFE function. Interestingly, we have not been able to decouple heparin binding from biological activity or from BMP binding, suggesting a possible prominent role of heparin in the ERFE mechanism of action.
Finally, we used our mutagenesis data to determine the minimal region required for ERFE bioactivity. Peptides that contain only one of the BMP-binding domains no longer suppress hepcidin despite retaining a partial ability to bind BMPs. Thus, cooperation between multiple BMP-binding domains in the N-terminal extended region are likely needed for hepcidin suppression. These structural data will help define the mechanism of ERFE action and guide future drug design, as ERFE inhibition may be a powerful tool for the treatment of iron overload in hemoglobinopathies like β-thalassemia.
Disclosures
Nemeth:Ionis: Consultancy; RallyBio: Consultancy; Intrinsic LifeSciences: Current equity holder in private company; Vifor: Consultancy; Disc Medicine: Consultancy; Protagonist: Consultancy; Silarus: Current equity holder in private company; Novo Nordisk: Consultancy; GSK: Consultancy; AstraZeneca: Consultancy; Shield Therapeutics: Consultancy. Ganz:Gossamer Bio: Consultancy; Incyte: Consultancy; Vifor: Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Consultancy; ADARx: Consultancy; Silence Pharma: Consultancy; Alnylam: Consultancy; Rockwell: Consultancy; Pharmacosmos: Other: Invited Speaker; Sierra Oncology: Consultancy; Disc Medicine: Consultancy; Akebia: Consultancy; Ionis: Consultancy; Intrinsic LifeSciences: Consultancy, Current equity holder in private company.
Author notes
*Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal